Optimize Your Research
Competitive ELISA Kit
User Instruction
Human Vitamin B6, VB6 ELISA Kit
Cat.No: EA0132Hu
CONTENTS
Precision
Intra-assay Precision (Precision within an assay) Three samples of known concentration were tested on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays) Three samples of known concentration were tested in separate assays to assess inter-assay precision.
CV (%) = SD/mean x 100
Intra-assay: CV< 10%
Inter-assay: CV< 12%
Inter-assay Precision (Precision between assays) Three samples of known concentration were tested in separate assays to assess inter-assay precision.
CV (%) = SD/mean x 100
Intra-assay: CV< 10%
Inter-assay: CV< 12%
Standard curve range: 3.75-240nmol/L
Sensitivity: 1.69nmol/L
Size: 96T
Storage: Store at -20°C for one year. Or store at 2-8°C for 6 months. If individual reagents are opened it is recommended that the kit be used within 1 month. Avoid repeated thaw cycles.
Intended Use
This Competitive ELISA kit is for the quantification of Human Human Vitamin B6 (also known as VB6) in serum, plasma, cell culture supernatants, urine, tissue homogenates or other biological fluids.
Note: This product is for research use only, not for use in diagnosis procedures. It’s highly recommended to read this instruction entirely before use.
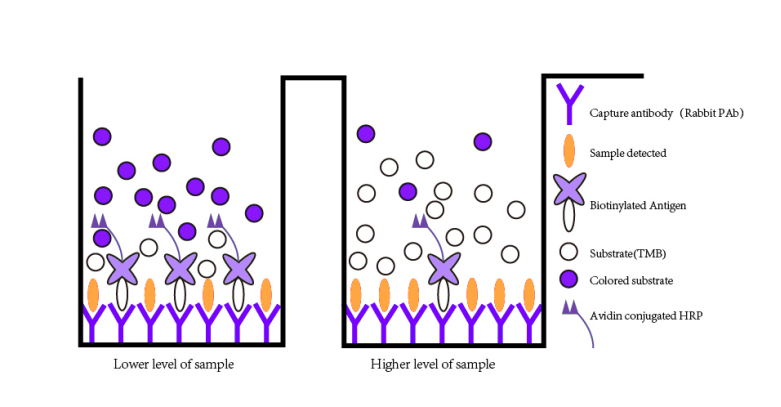
Assay Principle
This kit is an Enzyme-Linked Immunosorbent Assay (ELISA). Add samples to the pre-coated plate. Then add biotinylated antigen. The antigens in the samples compete with the biotinylated antigen to bind to the capture antibody and incubate. Unbound antigen is washed away during a washing step. An avidin-HRP is then added and then incubate. Unbound avidin-HRP is washed away during a washing step. TMB Substrate is then added and color develops. The reaction is stopped by addition of acidic stop solution and color changes into yellow that can be measured at 450 nm. The intensity of the color developed is inversely proportional to the concentration of VB6 in the sample. The concentration of VB6 in the sample is then determined by comparing the O.D. of the samples to the standard curve.
| Reagent Provided | |
| Components | Quantity |
| Pre-coated plate | 12 * 8 well strips x 1 |
| Human VB6 Standard, lyophilized | 2 vials |
| Standard/Sample diluent | 6ml ×1 vial |
| Biotinylated antigen, lyophilized | 1 vial |
| Avidin-HRP concentrate | 100ul × 1 vial |
| Biotinylated antigen diluent | 6ml x1 |
| Avidin HRP diluent | 5.9ml × 1 vials |
| Substrate solution A | 6ml x1 |
| Substrate solution B | 6ml x1 |
| Stop solution | 6ml x1 |
| Wash buffer (25x) | 20ml × 1 vial |
| Plate sealer | 2 pics |
| User instruction | 1 |
Material Required but Not Supplied
- 37°C±0.5°C incubator
- Precision pipette and disposable tip
- Deionized or distilled water
- Clean tubes
- Absorbent paper
- Microplate reader with 450 ± 10nm wavelength filter
Precautions
- Prior to running the assay, the kit and sample should be warmed naturally to room temperature 30 minutes.
- Once the desired number of strips has been removed, immediately reseal the bag to protect the remain from deterioration. Cover all reagents when not in use.
- Make sure pipetting order and rate of addition from well-to-well when pipetting reagents.
- There are two vials of standard in the kit for users, please cover the other unused vial and keep refrigerated.
- Do not allow wells to become dry during the assay procedure.
- This instruction should be strictly followed in the experiment.
- Pipette tips and plate sealer in hand should be clean and disposable to avoid cross-contamination.
- Avoid using the reagents from different batches together.
- Substrate solution B is sensitive to light, don’t expose substrate solution B to light for a long time.
- Stop solution contains acid. Please wear eye, hand and skin protection when using this material. Avoid contact of skin or mucous membranes with kit reagent.
- The kit should not be used beyond the expiration date.
Specimen Collection
Serum Allow serum to clot for 10-20 minutes at room temperature. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Plasma Collect plasma using EDTA or heparin as an anticoagulant. After mix 10-20 minutes, centrifuge samples for 20 minutes at 2000-3000 RPM. Collect the supernatant without sediment.
Cell culture supernatant Collect by sterile tubes. When detecting secrete components, centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatants. When detecting the components in the cell, use PBS (pH 7.2-7.4) to dilute cell suspension, the cell concentration of approximately 1 million/ml. Damage cells through repeated freeze-thaw cycles to let out the inside components. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Tissue Rinse tissues in ice-cold PBS (pH 7.4) to remove excess blood thoroughly and weigh before homogenization. Mince tissues and homogenize them in PBS (tissue weight (g): PBS (mL) volume=1:9) with a glass homogenizer on ice. To further break down the cells, you can sonicate the suspension with an ultrasonic cell disrupter or subject it to freeze-thaw cycles. The homogenates are then centrifuged for 15 minutes at 12,000 RPM at 4°C to get the supernatant. Avoid freeze/thaw cycles.
Urine/Ascites/Cerebrospinal fluid Collect by sterile tube. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Plasma Collect plasma using EDTA or heparin as an anticoagulant. After mix 10-20 minutes, centrifuge samples for 20 minutes at 2000-3000 RPM. Collect the supernatant without sediment.
Cell culture supernatant Collect by sterile tubes. When detecting secrete components, centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatants. When detecting the components in the cell, use PBS (pH 7.2-7.4) to dilute cell suspension, the cell concentration of approximately 1 million/ml. Damage cells through repeated freeze-thaw cycles to let out the inside components. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Tissue Rinse tissues in ice-cold PBS (pH 7.4) to remove excess blood thoroughly and weigh before homogenization. Mince tissues and homogenize them in PBS (tissue weight (g): PBS (mL) volume=1:9) with a glass homogenizer on ice. To further break down the cells, you can sonicate the suspension with an ultrasonic cell disrupter or subject it to freeze-thaw cycles. The homogenates are then centrifuged for 15 minutes at 12,000 RPM at 4°C to get the supernatant. Avoid freeze/thaw cycles.
Urine/Ascites/Cerebrospinal fluid Collect by sterile tube. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Note
- The supplier is only responsible for the kit itself, but not for the samples consumed during the assay. The user should calculate the possible amount of the sample used in the whole test
- Samples to be used within 5 days should be stored at 2-8°C. Samples should be aliquoted or must be stored at -20°C within 1 month or -80°C within 3 months. Avoid repeated freeze thaw cycles.
- Samples should be brought to room temperature before starting the assay.
- Samples containing NaN3 can’t be tested as it inhibits the activity of Horse Radish Peroxidase (HRP).
- Collect the supernatants carefully. When sediments occurred during storage, centrifugation should be performed again.
- Hemolysis can greatly impact the validity of test results. Take care to minimize hemolysis.
Reagent Preparation
- All reagents should be brought to room temperature before use.
-
Standard Reconstitute one vial of standard with 150ul of Standard/Sample diluent to generate a 480 nmol/L standard stock solution which should be used within 24 hours. Allow the standard to sit for 15 minutes with gentle agitation prior to making dilutions. Prepare duplicate or triplicate standard points by serially diluting the standard stock solution 1: 2 with diluent to produce 240 nmol/L, 120 nmol/L, 60 nmol/L, 30 nmol/L and 15 nmol/L solutions. Add Standard/Sample diluent only as the zero standard (0 nmol/L).
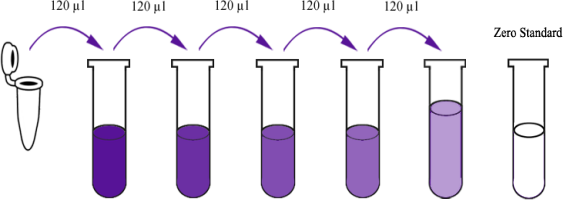
| Stock solution | 480 nmol/L | 240 nmol/L | 120 nmol/L | 60 nmol/L | 30 nmol/L | 15 nmol/L |
- Biotinylated Antigen Briefly centrifuge the biotinylated antigen vial then add 1ml Biotinylated antigen diluent to mix well. And then pipette all this solution back into the Biotinylated antigen diluent vial to mix well and generate a 6ml stock solution. Allow to sit for 10 minutes with gentle agitation prior to making dilutions.
- Avidin-HRP Concentrate Briefly low- speed centrifuge the avidin-HRP Concentrates solution and then pipette all avidin-HRP into the Avidin HRP Diluent vial. Mix well to generate a 6ml stock solution. Allow to sit for 10 minutes with gentle agitation prior to making dilutions.
- Wash Buffer Concentrate 25x Dilute 20ml of concentrated wash buffer with 480ml double distilled water to prepare 500 ml of wash buffer. If crystals have formed in the concentrate, warm it in a 40°C water bath and mix it gently until the crystals have completely dissolved.
Assay Procedure
- Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature.
- Determine the number of strips required for the assay. Insert the strips in the frames for use. The unused strips should be stored at 2~8°C for up to one month.
- Blank wells: Only add substrate solution A , substrate solution B and Stop solution as blank control.
- Add 50 ul diluted standard to standard well,add 50ul sample (Sample recommended dilution: 2-5 times when necessary) to the sample well, and add 50 ul biotinylated antigen to each well. Mix well. Cover the plate with a sealer and incubate for 60 minutes at 37°C.
- Remove the sealer and the liquid in the well, wash five times with 300 ul wash buffer manually. Invert the plate each time and decant the contents; hit 4-5 times on absorbent material to complete remove liquid. For automated washing, aspirate all wells and wash 5 times with wash buffer. Blot the plate on absorbent material.
- Add 50 ul avidin-HRP to the standard well and sample well, cover the plate with a sealer and incubate for 60 minutes at 37°C.
- Remove the sealer and wash as described above.
- Add 50 ul substrate solution A to each well and then add 50 ul substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark.
- Add 50 ul Stop Solution to each well, the blue color will change into yellow immediately.
- Determine the optical density (OD value) of each well immediately using a microplate reader set to 450 nm within 10 minutes after adding the stop solution.
Summary
| Prepare all reagents, samples and standards. | ||
| Add samples, standard and biotinylated antigen. Incubate for 60 minutes at 37°C. | ||
| Aspirate and wash 5 times. | ||
| Add avidin-HRP and incubate for 60 minutes at 37°C. | ||
| Aspirate and wash 5 times. | ||
| Add substrate solution A and substrate solution B and incubate in the dark for 10 minutes at 37°C. | ||
| Add stop solution. | ||
| Read the OD value within 10 minutes at 450nm. | ||
| Add 50ul stop solution. |
Calculation of Result
Average the duplicate readings for each standard, control, and sample. Create a standard curve by plotting the mean absorbance for each standard on the Y-axis against the target antigen concentration on the X-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the target antigen concentration on the X axis versus the O.D. of the standards on the Y axis and the best fit line can be determined by regression analysis. The linear equation (X = Y + Calibration Value) can be used to calculate the standard curve where X is the log of the concentration of the standard and Y is the OD value of the standard. If samples have been diluted (2-5 times is recommended), the concentration read from the standard curve must be multiplied by the dilution factor.
| Concentration | Blank | S5 | S4 | S3 | S2 | S1 | S0 |
| OD value | VB | V5 | V4 | V3 | V2 | V1 | V0 |
| Calibration value | VB-VB | V5-VB | V4-VB | V3-VB | V2-VB | V1-VB | V0-VB |
Typical Data
This standard curve is only for demonstration purposes. A standard curve should be generated with each assay.
| Troubleshooting | |
| High Background possible case | Solution |
| Improper washing | Increasing duration of soaking steps |
| Incorrect incubation temperature | Incubate at 37°C |
| Incubation time too long | Reduce incubation time |
| Substrate exposed to light prior to use | Keep substrate in a dark place |
| Substrate was contaminated | Replace substrate. Substrate should be clean and avoid crossed contamination by using the sealer |
| Contaminated wash buffer | Use a clean buffers and sterile filter |
| Weak or No Signal possible case | Solution |
| A reagent or a step of the procedure omitted by mistake | Check protocol and follow steps carefully |
| Antibody are not enough | Increase the concentration of the antibody |
| Improper washing | Increasing duration of soaking steps |
| Reagent are contaminated | Use new one |
| Pipette are not clean | Pipette should be clean |
| HRP was not added | Add HRP according to the instruction |
| Sample contains sodium azide | Don't prepare samples with sodium azide |
| Wrong incubation time or temperature | Check and follow protocol. Place plates in an incubator during incubation periods (set to 37ºC). |
| Poor Precision possible case | Solution |
| Pipetting error | Dispense quickly and identically into the side of each well. Use calibrated pipettes. |
| Incomplete washing | Make sure wells are washed adequately by filling the wells with proper amount of wash buffer. |
| Unclean wells | Inspect wells and remove debris prior to use. Wipe the outer bottom of plate clean to remove any debris or fingerprints prior to reading. |
| Poor standard curve possible case | Solution |
| Incorrect preparation of standard | Reconstitute standard as suggested on data sheet. |
| Capture Antibody did not bind | Use BT LAB ELISA plate in the kit |
| Inefficient washing | Be sure wash apparatus is working properly (i.e. distributing even volumes into each well). Be sure wells are empty after aspiration, yet be sure to fill wells in a timely manner. |
| Pipetting error | Dispense quickly and identically into the side of each well. Use calibrated pipettes. |
| Incorrect storage of components | Store all components as recommended on data sheet. Do not allow reconstituted reagents to stay at room temperature for excess time. |
Assay Principle