Protocols
Western Blotting protocol

Cell lysate
1. Place the cell culture dish in ice and wash the cells with ice-cold PBS.
2. Drain the PBS, then add ice-cold lysis buffer (1 ml per 107cells/100mm dish/150cm 2 flask; 0.5ml per 5x106 cells/60mm dish/75cm2 flask).
3. Scrape adherent cells off the dish using a cold plastic cell scraper, then gently transfer the cell suspension into a pre-cooled microfuge tube.
4. Maintain constant agitation for 30 minutes at 4°C.
5. Centrifuge in a microcentrifuge at 4°C. You may have to vary the centrifugation force and time depending on the cell type; a guideline is 20 minutes at 12,000 rpm but this must be determined by the end-user (e.g. leukocytes need a very light centrifugation).
6. Gently remove the tubes from the centrifuge and place on ice, aspirate the supernatant and place in a fresh tube kept on ice, and discard the pellet.
Tissue lysate
1. Dissect the tissue of interest with clean tools, on ice preferably, and as quickly as possible to prevent degradation by proteases.
2. Place the tissue in round-bottom microfuge tubes or Eppendorf tubes and immerse in liquid nitrogen to "snap freeze". Store samples at -80°C for later use or keep on ice for immediate homogenization. For a ~5 mg piece of tissue, add ~300 μl lysis buffer rapidly to the tube, homogenize with an electric homogenizer, rinse the blade twice with another 2x 300 μl lysis buffer, then maintain constant agitation for 2 hours at 4°C (e.g place on an orbital shaker in the fridge). Volumes of lysis buffer must be determined in relation to the amount of tissue present (protein extract should not be too diluted to avoid loss of protein and large volumes of samples to be loaded onto gels. The minimum concentration is 0.1 mg/ml, optimal concentration is 1-5 mg/ml).
3. Centrifuge for 20 min at 12000 rpm at 4°C in a microcentrifuge. Gently remove the tubes from the centrifuge and place on ice, aspirate the supernatant and place in a fresh tube kept on ice; discard the pellet.

Electrophoresis
1. Preparation of PAGE gels. Polyacrylamide gels are formed from the polymerization of two compounds, acrylamide and N,N-methylenebisacrylamide.
2. Preparation of samples for loading into gels. To denature, use a loading buffer with the anionic denaturing detergent sodium dodecyl sulfate (SDS), and boil the mixture at 95 -100°C for 5 minutes.
3. Load 20-40 ug total protein per mini-gel well. Note: Take care not to poke the well bottom with the tip as this will create a distorted band. Never overfill wells. This could lead to poor data if samples spill into adjacent wells, and poorly resolved bands.
4. When the dye molecule reaches the bottom of the gel, the power is turned off. Proteins will slowly elute from the gel at this point, so do not store the gel; proceed immediately to transfer.
Transfer of proteins
1. Two types of membranes are available: nitrocellulose and PVDF.
2. Transfer can be done in wet or semi-dry conditions. In wet transfer, the gel and membrane are sandwiched between sponge and paper(sponge/gel/membrane) and all are clamped tightly together after ensuring no air bubbles have formed between the gel and membrane. The sandwich is submerged in transfer buffer to which an electrical field is applied. The negatively-charged proteins travel towards the positively-charged electrode, but the membrane stops them, binds them, and prevents them from continuing on.
3. In semi-dry transfer, a sandwich of paper/gel/membrane wetted in transfer buffer is placed directly between positive and negative electrodes (cathode and anode respectively). Tips: As for wet transfer, it is important that the membrane is closest to the positive electrode and the gel closest to the negative electrode. The proportion of Tris and glycine in the transfer buffer is not necessarily the same as for wet transfer.
Blocking the membrane
1. Two blocking solutions are traditionally used: non-fat milk or BSA. Some antibodies give a stronger signal on membranes blocked with BSA as opposed to milk for unknown reasons.
2. To prepare a 5% milk or BSA solution, weigh 5 g per 100 ml of Tris Buffer Saline Tween20 (TBST) buffer. Mix well and filter.
3. Incubate for 1 hour at room temperature or overnight at 4°C agitation. Rinse for 5 seconds in TBST after the incubation.
Incubation with the primary antibody
1. Dilute primary antibody in TBST at the suggested dilution.
2. Incubate the membrane with diluted primary antibody for 1 hour at 37°C, or 2 hours at room temperature, or overnight at 4°C with agitation.
3. Remove antibody solution. Wash the membrane 3 times for 5-10 minutes each time at room temperature in TBST (50mM Tris, 100mM NaCl, 0.05% Tween-20, pH 7.6) with agitation. Note: Increase the concentration of Tween-20 to 0.1% reduces the background and increases the specificity, but it will reduce the sensitivity.
Incubation with secondary antibody
1. Incubate membrane with secondary HRP-conjugated diluted (according to manufacturer's instructions) in TBST for 1 hour at room temperature with shaking.
2. Remove antibody solution. Wash the membrane times for 5-10 minutes each time at room temperature in TBST (50mM Tris, 100mM NaCl, 0.05% Tween-20, pH 7.6) with agitation.
Chemiluminescent Reaction
1. Prepare and use the Chemiluminescent substrate according to the manufacturer's instructions.
2. Immediately wrap the membrane and expose to X-ray films for 10 second to 1 hour period. The exposure time may vary according to the mount of antibody and antigen.
ELISA protocol

Serum The samples should be allowed to clot in the collection tubes for a minimum of 30 minutes at room temperature. Serum should be separated from the clot by centrifuging the collection tube for 20 minutes at 2000 ~3000 RPM.
Plasma Collect plasma using EDTA or heparin as an anticoagulant. After mix 10 ~20 minutes, centrifuge samples for 20 minutes at 2000 ~3000 RPM. Collect the supernatant without sediment.
Saliva Collect saliva in a tube and centrifuge for 5 minutes at 2000 ~3000 RPM for 20 minutes. Collect the aqueous layer, assay immediately or aliquot and store samples at ≤ -20 °C. Avoid freeze/thaw cycles.
Cell culture supernatant Collect by sterile tubes. When detecting secrete components, centrifuge at 2000 ~3000 RPM for 20 minutes. Collect the supernatants. When detecting the components in the cell, use PBS (pH 7.2-7.4) to dilute cell suspension, the cell concentration of approximately 1 million/ml. Damage cells through repeated freeze-thaw cycles to let out the inside components. Centrifuge at 2000-3000 RPM for 20 minutes. Collect the supernatant without sediment.
Tissue Homogenates Rinse tissues in ice-cold PBS (pH 7.4) to remove excess blood thoroughly and weigh before homogenization. Mince tissues and homogenize them in PBS (tissue weight (g): PBS (mL) volume=1:9) with a glass homogenizer on ice. To further break down the cells, you can sonicate the suspension with an ultrasonic cell disrupter or subject it to freeze-thaw cycles. The homogenates are then centrifuged for 15 minutes at 12,000 RPM at 4 °C to get the supernatant. Avoid freeze/thaw cycles.
Urine Collect fresh urine into a sterile or disposable container. Centrifuge sample at 1,000 ~2,000 RPM for 5 minute. Assay immediately or aliquot supernatant and hold at -80°C. Avoid freeze/thaw cycles.
Bronchoalveolar lavage fluid/Synovial fluid Centrifuge samples for 15 minutes at 2000-3000 RPM to remove particulate. Collect the supernatant and freeze at -20°C.
If you're not sure about the specimen for your assay, please contact us to determine the optimal sample for your particular experiments.

The Standard solution
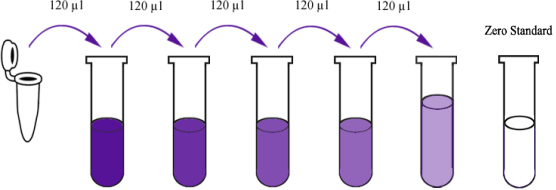
Reconstitute the 120μl of the standard with 120μl of standard diluent to generate a standard stock solution. Allow the standard to sit for 15 mins with gentle agitation prior to making dilutions. Prepare duplicate standard points by serially diluting the standard stock solution at ratio of 1:2 with standard diluent to produce solutions. Standard diluent serves as the zero standard (0 ng/ml). Any remaining solution should be frozen at -20°C and used within one month. Dilution of standard solutions suggested are as follows (e.g. for the detection of E3808Hu Standard concentration (12.8ng/ml) ):
|
1600ng/L |
Standard No.5 |
120μl Original Standard + 120μl Standard Diluent |
|
800ng/L |
Standard No.4 |
120μl Standard No.5 + 120μl Standard Diluent |
|
400ng/L |
Standard No.3 |
120μl Standard No.4 + 120μl Standard Diluent |
|
200ng/L |
Standard No.2 |
120μl Standard No.3 + 120μl Standard Diluent |
|
100ng/L |
Standard No.1 |
120μl Standard No.2 + 120μl Standard Diluent |
|
Standard Concentration |
Standard No.5 |
Standard No.4 |
Standard No.3 |
Standard No.2 |
Standard No.1 |
|
3200ng/L |
1600ng/L |
800ng/L |
400ng/L |
200ng/L |
100ng/L |
Wash Buffer
Dilute 20ml of Wash Buffer Concentrate 25x into deionized or distilled water to yield 500 ml of 1x Wash Buffer. If crystals have formed in the concentrate, mix gently until the crystals have completely dissolved.

Procedure
1. Determine the number of strips required for the assay. Insert the strips in the frames for use.
2. Add 50ul standard to standard well. Note: Don't add antibody to standard well because the standard solution contains biotinylated antibody.
3. Add 40ul sample to sample wells and then add biotinylated antibody to sample wells, then add 50ul streptavidin-HRP to sample wells and standard wells (Not blank control well). Mix well. Cover the plate with a sealer. Incubate 60 minutes at 37°C.
4. Remove the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0.35 ml wash buffer for 30 seconds to 1 minute for each wash.
Tips: For automated washing, aspirate or decant each well and wash 5 times with wash buffer. Blot the plate onto paper towels or other absorbent material.
5. Add 50ul substrate solution A to each well and then add 50ul substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark.
6. Add 50ul stop solution to each well, the blue color will change into yellow immediately.
7. Determine the optical density (OD value) of each well immediately using a microplate reader set to 450 nm within 10 minuets after adding the stop solution.

Biotinylated Antigen Preparation:Briefly centrifuge the biotinylated antigen vial then add 1ml biotinylated antigen diluent to mix well. And then pipette all this solution back into the biotinylated antigen diluent vial to mix well and generate a 6ml stock solution. Allow to sit for 10 minutes with gentle agitation prior to making dilutions.
Avidin-HRP Concentrate: Briefly low- speed centrifuge the avidin-HRP Concentrates solution and then pipette all avidin-HRP into the avidin HRP Diluent vial. Mix well to generate a 6ml stock solution. Allow to sit for 10 minutes with gentle agitation prior to making dilutions.
Procedure
2. Blank well: Only add substrate solution A , substrate solution B and stop solution as blank control.
3. Add 50ul diluted standard to standard well, add 50ul sample to the sample well, and add 50ul biotinylated antigen to each well. Mix well. Cover the plate with a sealer and incubate for 60 minutes at 37°C. Tips: Sample recommended dilution: 2-5 times when necessary.
4. Remove the searler and the liquid in the well, wash five times with 300ul wash buffer manually. Invert the plate each time and decant the contents; hit 4-5 times on absorbent material to complete remove liquid. Tips: For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate on absorbent material.
5. Add 50ul avidin-HRP to the standard well and sample well, cover the plate with a sealer and incubate for 60 minutes at 37°C.
6. Remove the sealer and wash as described above.
7. Add 50ul substrate solution A to each well and then add 50ul substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark.
8. Add 50ul stop solution to each well, the blue color will change into yellow immediately.
9. Determine the optical density (OD value) of each well immediately using a microplate reader set to 450 nm within 10 minutes after adding the stop solution.

Procedure
2. Set a Blank well without any solution.
3. Add 50ul negative control to each of the negative control wells and 50ul positive control to each of the positive control wells. Add 40ul sample diluent and then add 10ul sample to the sample well, mix well.
4. Cover with a plate sealer, and incubate for 30 minutes at 37°C.
5. Remove the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0.35 ml wash buffer for 30 seconds to 1 minute for each wash.
Tips: For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate onto paper towels or other absorbent material.
6. Add 50ul HRP to each well (except blank well). Cover with a plate sealer, and incubate for 30 minutes at 37°C.
7. Remove the sealer and wash as 5 times with wash buffer.
8. Add 50ul substrate solution A to each well and then add 50ul substrate solution B to each well. Mix well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark.
9. Add 50ul stop solution to each well, the blue color will change into yellow immediately.
10. Determine the optical density (OD value) of each well immediately using a microplate reader set to 450 nm within 15 minutes after adding the stop solution.
Immunohistochemistry (IHC)

Procedure
2.Heat sections on the specimen slide to improve adherence. Put the specimen slide in the water bath and heat at 65°C 30 min.
3.Remove paraffin and rehydrate the tissue
Place the slides in a rack, and perform the following washes:
a. Xylene: 3 x 3 minutes.
b.100% ethanol: 3 minutes.
c. 95% ethanol: 3 minutes.
d. 85 % ethanol: 3 minutes.
e. 70 % ethanol: 3 minutes.
f. Running cold tap water to rinse.
4.Perform heat induced or protease induced epitope retrieval
Immerse slides into preheated Antigen Retrieval Solution (1x, 100°C) 10 minutes. Remove slides from the water bath, and let it cool to room temperature. Gently rinse the slides with PBS 3 times (3 minutes each time).
5.Block endogenous peroxidases, phosphatases (for enzymatic labels) and biotin (when using biotin/avidin systems) Put the slides in 3% H2O2-methanol solution (30% H2O2: 100% methanol=1: 9) at room temperature 15 min, and gently rinse the slides with PBS 3 times (3 minutes each time).
6.Block non-specific binding sites
Put the slides in 5% non-fat milk at 37°C 5 min, and gently rinse the slides with PBS 3 times (3 minutes each time).
7.Incubate with primary antibody
Put the slides in the wet box, and add proper volume of primary antibody diluent, then incubate at 37°C 60 min, and gently rinse the slides with TBST 3 times then PBS (3 minutes each time).
8.Incubate with secondary antibody
Add proper volume of secondary antibody diluent, then incubate at 37°C 10 min, and gently rinse the slides with TBST 3 times then PBS (3 minutes each time).
9.Incubate with amplification reagent
Add proper volume of avidin diluent, then incubate at 37°C 10 min, and gently rinse the slides with TBST 3 times then PBS (3 minutes each time).
When performing experiments with multiple fluorescent labels, ensure that each fluorophore can be spectrally separated. This ensures that one fluorophore does not get detected in another fluorophore’s channel. For this purpose, we recommend mocking up the fluorophore excitation and emission spectra with the help of a spectrum viewer at the experimental design stage.
10.Incubate with DAB or other substrate solution (for enzymatic labels only)
Add 1–5 drops of DAB chromogen solution to cover the entire tissue section and incubate for 3–5 minutes, and rinse in deionized H2O and drain the slides.
11.Counterstain
Add 1–5 drops of hematoxylin to cover the entire tissue section and incubate for 5 minutes, and rinse in deionized H2O and drain the slides.
12.Dehydrate tissue sections (only needed when organic mounting media are used)
Cover stained tissue with a coverslip of an appropriate size. Place slides vertically on a filter paper or towel to drain excess mounting medium and allow them to dry.
13.Mount coverslip
Add drops of resinene, then seal with coverslip. Visualize tissue under a light microscope.
